Abstract
Introduction. Thalassemia Syndromes (TS) are currently categorized as transfusion dependent (TDT, primarily Thalassemia Major patients) and non transfusion dependent (NTDT, primarily Thalassemia Intermedia patients). TDT includes patients who need regular transfusions for survival with at least ≥7 ml/kg/month of packed red blood cells. NTDT is a term used to label patients who do not require lifelong regular transfusions for survival, although they may require occasional or even frequent transfusions in certain clinical settings and usually for defined periods of time. Therefore, the current TS classification suggests two defined classes of disease severity, TDT and NTDT, with different prognosis. However, recent survival studies in Western and Eastern countries showed that life expectancy of TDT is today quite similar to that of NTDT (Vitrano et al. Br J Haematol. 2017; Rajaeefard et al. Epidemiol Health 2015). The main aim of this study was to revisit the dichotomous classification of TS towards a classification including a "continuum" of the same disease divided in multiple stages according to the severity of phenotypes.
Methods. This was a retrospective study on patients with TDT and NTDT, born after February 13, 1965 (data of approval of Deferoxamine chelation treatment), and attending 9 Italian centres. Our Ethical Committee approved the protocol on May 25, 2017. Focusing on clinical severity indicators, a cluster analysis was performed to explore if the current TS classification fits with two classes of risk, regardless if the patients had TDT or NTDT. The following indicators of clinical severity were selected: 1) age at first transfusion, years; 2) age at starting chelation, years; 3) transfusion therapy (yes/no); 4) Mean serum ferritin levels, ng/ml; 5) number of complications. Cluster analysis was applied at these indicators with the task of grouping a set of statistical units in such a way that units in the same group (cluster) will be more similar to each other than to those in other clusters. If current classification fits with clinical severity of TS, only two classes of risk matching TDT and NTDT should be identified.
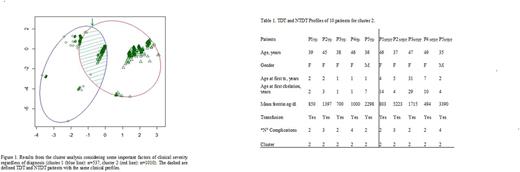
Results. Overall 1547 patients, 1332 with TDT (mean age 35.6±0.2 years) and 215 with NTDT(mean age 38.4±0.5years), were included in the study. Mean age at first transfusion was 1.5±0.04 years and 8.4±9.4 years in TDT and NTDT, respectively.Mean age at starting chelation was 4.6±0.1 years in TDT and 13.6±11.4 years in NTDT. Splenectomy was more common in NTDT (80.2%) than TDT ( 51.9%). Diabetes, heart failure and hypogonadism were most frequent in TDT, while cirrhosis was equally distributed. Figure 1 shows results from cluster analysis suggesting that it was not possible to classify TS only into two separate groups. The two obtained clusters intersect an area were patients with TDT and NTDT had very similar clinical features. Patients could not be divided by their clinical profiles between TDT or NTDT distinct groups (Fig. 1). Table 1 shows some TDT and NTDT patients allocated in two different clusters but with very similar clinical profiles.
Conclusions. Cluster analysis suggests that the classes of risk of TDT and NTDT may not fit with current classification of TS. Indeed, some patients are detected in the same area of the clusters, independently from onset of diagnosis. This could be due to the presence of a "continuum" phenotype from NTDT to TDT. However, an effect of conventional treatment in improving phenotype of patients with TDT could be not excluded. Therefore, to validate this hypothesis, these data have to be confirmed in a larger population, encompassing different intensity of conventional treatment. In conclusion, although these results call for a potential revision of the clinical classification of thalassemia based on strict categories of severity towards a classification including a "continuum" of the same disease divided into one that manifests in categories (Classes of Risk), this hypothesis must be validated with larger setting of patients and discussed inside of International Working Group of expert opinion leaders on this field.
Pepe: Chiesi Farmaceutici and ApoPharma Inc.: Other: Alessia Pepe is the PI of the MIOT project, that receives no profit support from Chiesi Farmaceutici S.p.A. and ApoPharma Inc.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal